Cardiogenic shock
Lessons from DanGer-Shock and ECLS-Shock Trials

Jeong Hoon Yang, MD, PhD
Sungkyunkwan University, KoreaRecently, the application of mechanical circulatory support devices has been increasing in patients with profound cardiogenic shock. However, randomized controlled trials comparing the clinical outcomes between mechanical circulatory support devices and usual standard treatment have been conducted on a small, limited number of patients, and these also showed no difference between the two groups. Recently, the ECLS-SHOCK, DanGer Shock trials, a large-scale randomized controlled trial comparing the effects of mechanical circulatory support devices and conventional standard treatment in patients with cardiogenic shock complicating acute myocardial infarction, has been published. The ECLS-SHOCK trial was conducted in 420 patients from 44 centers from 2019 to 2022, and treatment using extracorporeal membrane oxygenator (ECMO) failed to lower the 30-day mortality rate compared to standard treatment. Furthermore, the frequency of bleeding complications and vascular complications was significantly higher in the ECMO group. This study showed the limitations of low rates of active use of ECMO before primary percutaneous coronary intervention and left ventricular unloading was performed in only 6% of the ECMO group, and cardiac replacement therapy was performed in limited patients. In contrast, the DanGer Shock trial first demonstrated the clinical benefit of the mechanical circulatory support device by significantly reducing the 6-month mortality rate in the Impella group compared to the standard treatment group. Similarly, the complications of bleeding and lower limb ischemia increased in the Impella group. However, as the title of the DanGer Shock study suggests, the study started in Denmark and had difficulty to enroll study population, so it was possible to complete the study over a long enrollment period of 10 years by enrolling in Germany and the UK in the latter half. Unlike the clinical benefits of patients enrolled in Denmark, the lack of clinical benefits in patients enrolled in Germany and the UK may reveal limitations in the generalization of the study. However, unlike the ECLS-SHOCK trial, this is the first randomized study of mechanical circulatory assist devices that reduced the mortality rate under conditions that minimized the possibility of death due to brain dysfunction by excluding out-of-hospital cardiac arrest with a Glasgow coma scale score of less than 8, which has great clinical significance. If we conduct randomized controlled trials with a well-designed protocol including prevention of the mechanical circulatory devices-related complications commonly developed in the two trials and general critical care such as prevention and treatment of sepsis and acute renal injury, we can look forward to the promising results of randomized controlled trials related to mechanical circulatory assist devices that are currently underway.
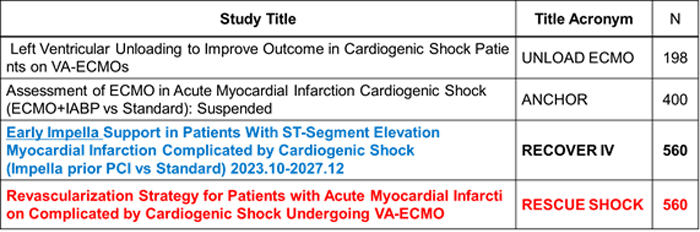
Table 1. Ongoing trials on mechanical circulatory support devices;
ECMO: extracorporeal membrane oxygenator, IABP: intraaortic balloon pump,
PCI: percutaneous coronary intervention, VA-ECMO: veno-arterial extracorporeal membrane oxygenator
